In a research project in my laboratory at the University of Basel, analysis of caveolae has clarified their composition in lung endothelia isolated by a silica procedure from Rattus norvegicus . This led to the surprising finding that, despite the predicted role in constitutive vesicular traffic, no cargo receptors could be found, and that the machinery presumably corresponded to factors that were known from mechanotransduction in other eukaryotic organisms and was likely related to blood pressure regulation in humans and rat (see ). Further claims demonstrating the fission of caveolae in an in vitro assay and showing the release from the plasmalemma could not be confirmed using the same procedure and, however, an energy requirement for the intactness of the plasma membrane could be established in my own studies shown here (see Webpage).
Surprisingly, and not yet connected to caveolae function, I found the significant similarity of a phosphatidylinositol-transfer protein to caveolin-1 (Cav1) (see Webpage) that, once modeled by a loop procedure, showed two cholesterol or lipid binding sites that were coupled and included the surface binding site(s) that had been previously identified in Cav1 (A, B) . In the attempt to find the structural match of the Cav1 model with published partial structural analyses of Cav1 peptides there was no consistent explanation for the apparent clustering of caveolin by the "scaffolding domain" , that would correspond here to an extended structure (strand) followed by a loop wherein the majority of the former is predicted to be hidden in between the membrane-span and the rest of the currently modeled polypeptide. Further structural analyses would be required to test the possible extensive exposure of membrane-span residues as well as the dynamic changes of amino acids in proximity to these that could be predicted. Thus the role of caveolae in the vascular system may be explained by their biochemical composition including large quantities of reggies/flotillins (B, below), that are likely to form a caveolae coat, and may include hitherto known signaling proteins . Recently, reggie/flotillin-containing vesicles/membrane domains have been shown to be internalized in a process of "massive" endocytosis wherein a large fraction of the entire cell surface is turned over. It is hitherto unknown, whether a similar mechanism is involved in endocytosis in general that represents a physiological uptake, and whether the uptake of bacteria by caveolae is mechanistically analogous. Endothelial Cav1 is implicated in nitric oxide (NO) signaling wherein the extent and distance of diffusion of NO is one of the variables that has been widely discussed. Would the signaling molecule be transferred from cell to cell in a paracrine mode or would intracellular signaling suffice in vasocoupling of endothelial and muscle cells?
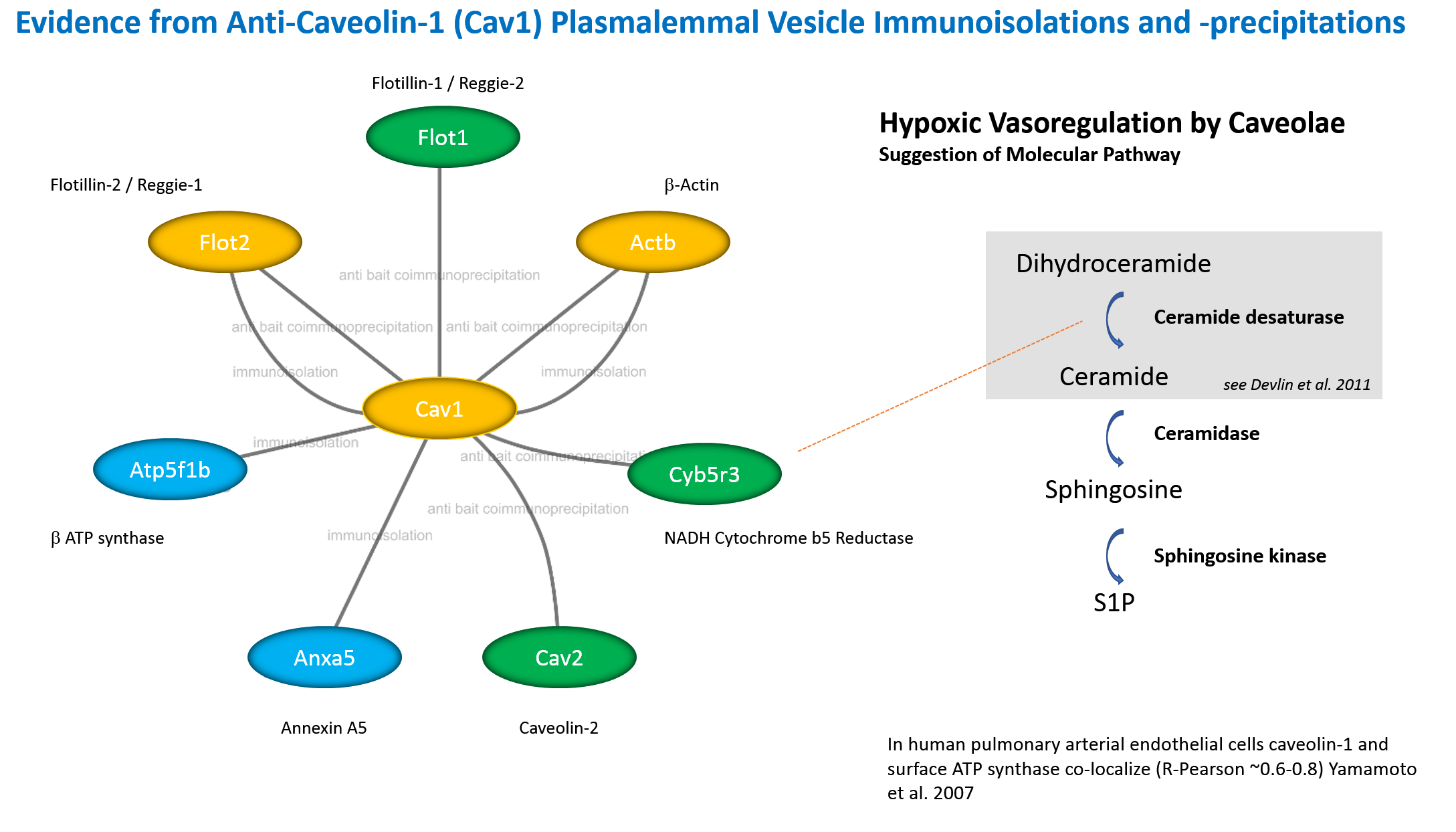
Interactome of Caveolin Analyzed by Immunopurifications. Perfusion procedure using silica to coat the endothelial plasma membrane from vascular beds (A). Immunoisolation of caveolae from silica purified membranes and identification of three major proteins in standard conditions (B). EM (C). Immunoisolation of caveolae from silica purified membranes, vesicle release by cytosol incubation mimicking a budding reaction (D). Analysis of budded vesicles by immunoisolation for ESA/reggie-1/flotillin-2 (E). Analysis of a "rough" luminal/apical membrane fraction from endothelia from lung obtained by the silica-coating shows that the abundant protein at approx. 45 kD corresponds to the elongation factor 1 α (EF1A) which now has been implicated in mechanosignalling by elucidating its function in cardiac hypertrophy (F) (Grund et al. 2019 EMBO Mol Med 11:e10018). EF1A1 interacts with TIP30 and is upregulated in response to pressure. Determination of the caveolin-1 (Cav1) complex stoichiometry from silica-purified membranes (vesicles, immunoisolation, detergent-extract/velocity sedimentation) (G). Summary of the immunoisolation of caveolae (H) from silica-purified membranes. Recent results suggest that ATP synthase subunit β (Atp5f1b) and NADH-cytochrome b5 reductase (Cyb5r3) are surprisingly found at the plasma membrane in endothelia, which has been shown by us and confirmed by several other laboratories. The caveolar proteins "cavins" were hitherto not found in our immunoisolates or co-immunoprecipitates (I). The interactome (considering the isolated and interacting proteins) of Cav1 in lung endothelia from apical plasmalemma (J). The abundant reggie-1/flotillin-2 (Flot2), β-actin (Actb) and Cav1α (Cav1) were demonstrated by immunopurification of intact vesicles (orange). The proteins shown in green (and flotillin-1/reggie-2, flotillin-2/reggie-1, β-actin) were co-immunoprecipitated from solubilized extracts derived from apical membranes bound to the silica pellicle. Proteins further visualized by immunopurification were ATP synthase F1 subunit β (Atp5f1b) and annexin A5 (Anxa5) (blue). Role in ATP synthesis of plasma membrane (mitochondrial-derived) ATP synthase (K). A neuronal role beyond endothelial function of plasma membrane ATP synthase (L). New intricacies: The secretory pathway and ATP synthase (M).
With the completion of sequencing of the rat genome, it seems likely, that previously we had identified neither a particular form of NAD(P)H cytochrome b5 reductase (Cyb5R3), one of which is likely N-terminally myristoylated, whereas the other contains an alternative N-terminal sequence generated by alternative splicing, nor had we de-identified a distinct isoform predicted to be non-microsomal in the human proteome. The biochemical characteristics of the protein that we had purified nevertheless suggested, that it was a microsomal enzyme that interacted with Cav1.
To our big surprise in 2012, Cyb5R3 that we found interacting with Cav1α, has been described as a regulator of NO signaling via reduction of α-hemoglobin expressed in endothelia of the human vasculature . In contradistinction to our suggestions on vasocoupling by mediators (ATP and other molecules/autacoids) these results of other researchers suggested, moreover, that cells were coupled via gap junctions that transmitted intracellular signals to the muscle cell layer. The elucidation of the role of Cyb5R3 and the transfer of electrons from NADH via Cyb5R3 to possibly multiple targets and the role of distinct isoforms awaits further analyses and may shed a new light on the general enzymology of lipid rafts and of caveolae in particular.
Recent results in structural analysis
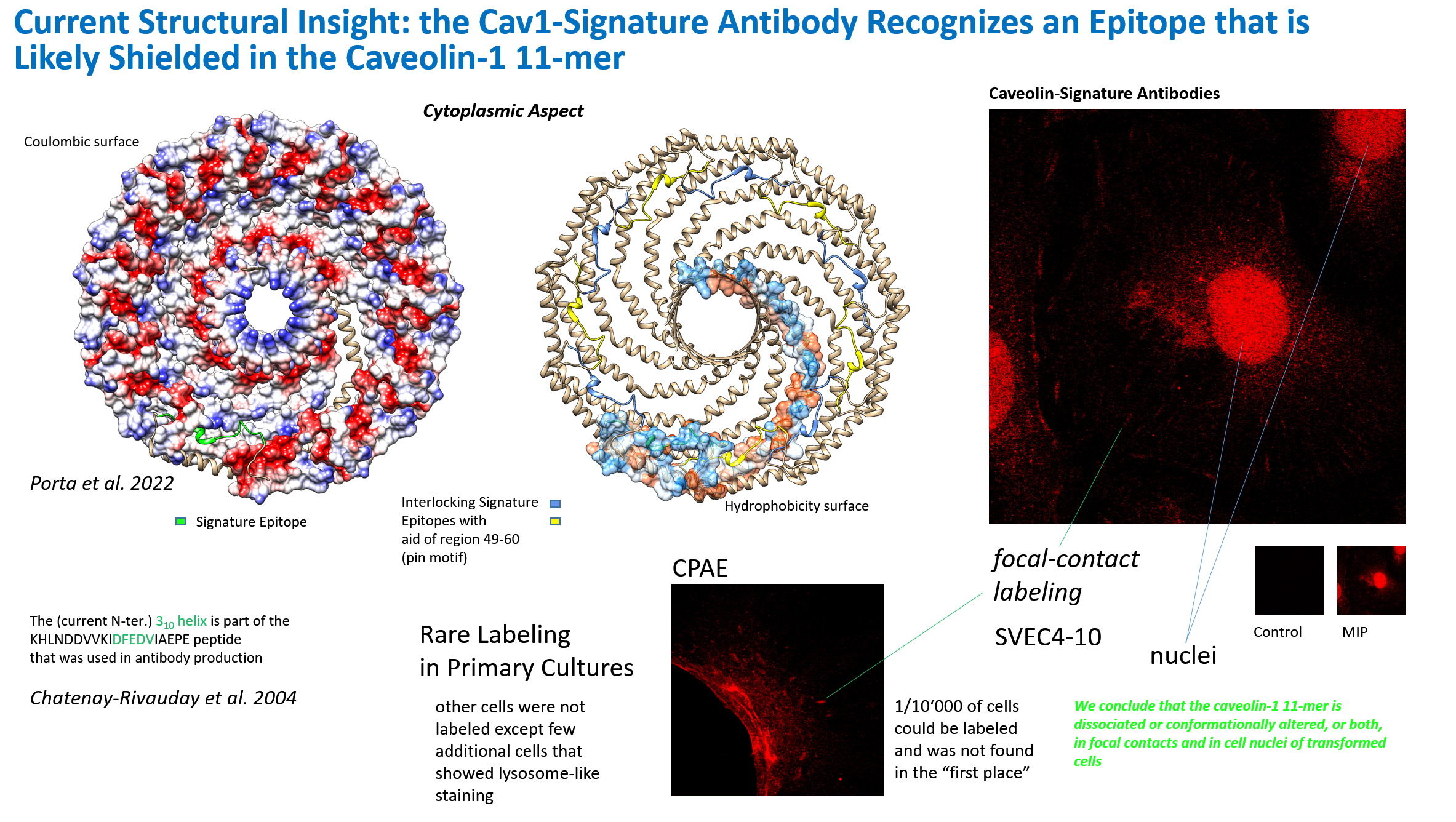
suggest that caveolin-1 is akin to a lipid-, ion- or other molecular channel with unknown novel features and a central pore within the oligomer of the 11 subunits found here in 2022. With the new structure in mind, a description of the signature-epitope antibody that I had generated at the
Biocenter, Basel and we had published in Chatenay-Rivauday et al. 2004
with respect to focal-contact type labeling and nuclear localization can now be found (N). A 3,10-helix is shown in the DFEDV sequence of the signature-motif, and possibly peptide that was used for raising the antibodies and could correspond to
a switchable region. Likely the signature-epitope region within the caveolin-1 molecule detected by the antibody is entirely shielded in classical plasmalemmal caveolae and only revealed in the cell nucleus, in mechanotransducing focal contacts and/or transduction-active contacts in
transformed cells.
Figures (A, B) shown above should now be considered outdated or could be revised when a cytoplasmic caveolin-lipoprotein particle or caveolin protein micelle is established. Our results on plasmalemmal vesicles in rat lungs suggested that vesicular caveolin-1
would constantly be released into solution to some small extent when these isolated domains were incubated and spun or re-spun in the centrifuge.
Sponsors 

References

Projects p24/TMED Structural Modeling Critical Issues on Caveolin Critical Issues on Carbohydrates Hypertensive Crisis Previous Work