Modeling of caveolin Klaus Fiedler
|
A |
|
Caveolin-1 was previously found to be
similar to PITPalpha (Fiedler, 2008).
The MODELLER algorithm was used here subjecting loops to modeling. Global
interaction of cholesterol and local CRAC binding was analyzed. An initial
loop was modeled and resulted in slight variation of the signature motif of
VIP-21 / caveolin-1 (within amino acids 60-80) (brown). A The final
results were generated with modeling residues 1-20, 44-86, 123-141, 152-178
and were tested for cholesterol and further docking of lipids. B
Cholesterol is shown as spherical molecule in the newly described binding
cavitas. CoA-acyl thioester bound to the binding cavity with intermediate to
high affinity. The primary binding hit is shown in global interaction. Part
of the surface has been sliced (clipped). C Putative binding interaction of VIP-21 to cholesterol in the
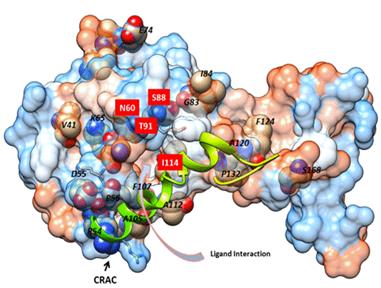
vicinity of the membrane span. D CRAC binding of cholesterol was shown
with primary hit within the binding cavity with local area interaction. The
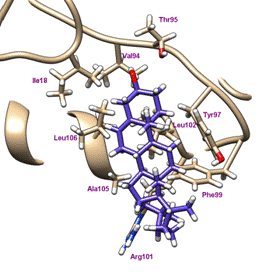
residues within 4 Å of the structure were labeled with Val94, Thr95, Tyr97,
Phe99, Arg101 and Leu102, Ala105, Leu106 extending into the membrane span of
caveolin and to Ile18 of the N-terminus. CRAC lipid binding was variable with
some hits and hydrophobic interactions on the backside of the molecule. E
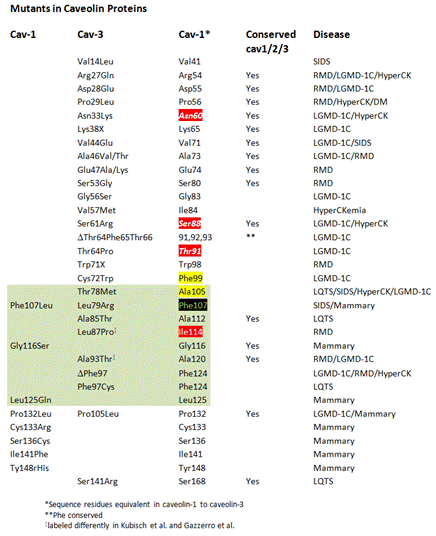
Disease Mapping onto the caveolin surface by analogy; the cholesterol binding
site is labeled. The CRAC-binding site on the backside of the 180° turned
molecule and the Y14 residue (src-kinase substrate) are not shown. The
disease causing mutations in caveolin-3 were indicated in equivalent
positions on the caveolin-1 protein after sequence comparison. Residues in
the proximity of cholesterol (4 Å) are shown with underlayed color. A full
summary of available data on natural gene variants is on Ensembl.org. Disease
mapping was done exclusively with reference to NP Refsequences.
|
Caveolin was previously shown to interact
with NADH-cytochrome B5 reductase in extract from lung apical endothelial
plasmalemma (Chatenay-Rivauday et al., 2004). See overview for putative involvement of the
cytochrome B5 reductase in the cholesterol biosynthetic pathway. CRAC site interactions will in the future be further analyzed for binding of cholesterol biosynthetic pathway intermediates. F
|
|
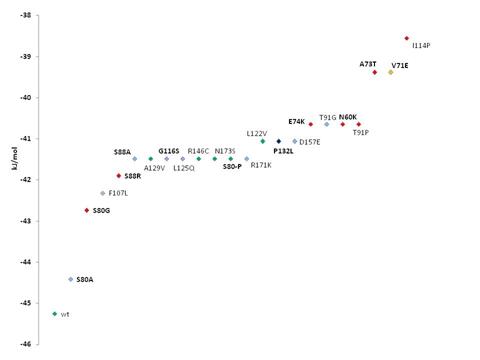
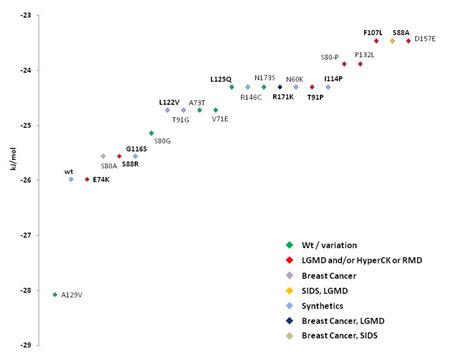
F Caveolin mutants implicated in
various diseases were measured in docking affinity to cholesterol. Binding of
cholesterol to the wildtype protein and mutants indicated in one-letter amino
acid code and position (caveolin-1 lettering). Green indicates wildtype or
variations of caveolin-1, red shows mutant proteins that are analogous to
structural replacement of caveolin-3 amino acid residues there implicated in
limb girdle muscular dystrophy (LGMD)(see Table II) and/or HyperCKemia
(HyperCK) or Rippling Muscle Disease (RMD). Violet indicates the breast
cancer implicated mutants of caveolin-1. Orange indicates the Sudden Infant
Death Syndrome (SIDS) / LGMD mutant within caveolin-3. Light blue shows
mutants synthetically constructed without known wildtype isoform or analogue
in caveolin-1 or -3 (see Ensembl variation tables cav-1 and cav-3). Breast
cancer mutant Pro132Leu analogous in sequence to caveolin-1 and to
caveolin-3, and involved in LGMD in caveolin-3, is indicated in dark blue.
The new breast tumor mutation Phe107Leu also implicated in SIDS is shown in
light brown. Binding was modeled with global cavity-docking (F) and local CRAC-docking (G). Caveolin-1 was compared by
homology search with caveolin-3 (Cav-1,
Cav-3) and RefSequence aligned
residues that are, due to 61.6% identity likely in equivalent locations in
structure, are indicated in column 3 (Cav-1).
Column 4 (Conserved Cav1/2/3) are
conserved residues. Cav-1 in the first column indicates disease mutations
suggested based on a recent analysis. |
|
|
|
|
B C |
|
|
||||||
|
D |
|
|
G
|
|
|
|
The Phe107Leu
mutation implicated in breast cancer in cav1 may interfere with the
structural coupling of the intermediate affinity cholesterol binding to CRAC
and the on-reaction of the main binding pocket; in other caveolins sterols of
a different type may require different coupling residues. It is interesting
to note that natural variants of caveolin show a higher affinity to
cholesterol if considering the established and newly modeled CRAC binding. I expect that
caveolin may require cholesterol for its travel through the cellular membrane
and cytoplasmic system – also the release from the membrane and
import/localization to the nucleus may implicate cholesterol. |
|
|
|
|
|
|
|
|
|||
|
|
|
|
||||||
|
|
|
|
||||||
|
|
|
|
||||||
|
|
|
|
||||||
|
|
|
|
|
|
|
|